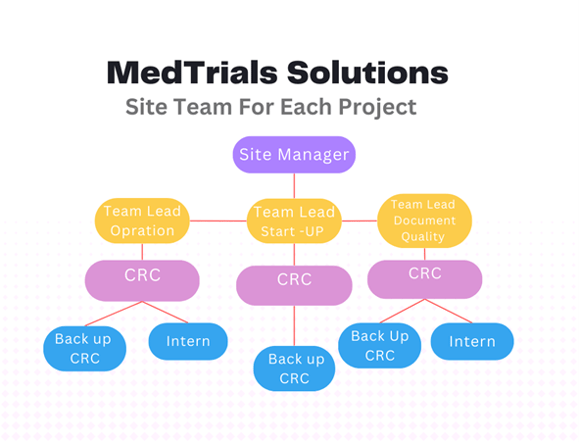
MedTrial
“Standards of Excellence”
MedTrial ’ “Standards of Excellence” criteria ensure that highly experienced clinical team members are working on your trial, to streamline the planning and start-up phase, create appropriate project plans to meet your study criteria, and quickly adapt to study challenges and changes in the regulatory environment.
MedTrials Solutions
Since 2010, MedTrials Solutions CRC team has been successfully providing services in many research sites of different clinical trial projects, in areas such as medical devices, chemical and biological products. Our therapeutic experience includes oncology, cardiovascular, anti-infection, autoimmune diseases, endocrine system disease, neural diseases, respiratory diseases, ophthalmology and digestive diseases. We focus on the following diseases: lung cancer, liver cancer, gastric cancer, Alzheimer’s disease, schizophrenia, epilepsy and diabetes mellitus. We have experience in clinical trials from Phase I to Phase IV, non-interventional outcome studies and medical device studies.


Oncology Expertise – Operations
With our CRC team’s specialized support, your projects can initiate earlier than the scheduled timeline and greatly increase enrollment speed – enabling timely and high-quality completion of data entry and significantly improved monitoring efficiency. Our superior CRC services are recognized by sponsors, investigators, study sites and other partner CROs. We have had no major findings at audits/quality control visits.


Our services
Project related training and internal meetings
Site Management
Study Start Up Activity
Coordination of site initiation
Management of research documents and files
Management of study drugs and supplies
Management of lab samples and auxiliary examination
Ethics committee communications and document submission


Site Management Service
Subject enrollment and training
Site administration service
Project training and status management
Audit and inspection preparation
Management of clinical trial supplies
· Project schedule management
· Subject training
· Subject management
· Laboratory management
· AE/SAE reporting and follow-up
· Source documents preparation
· CRF data entry and management
· Monitoring, Inspection, Audit preparation and response
· Conducting tasks related to Clinical trail
· Essential documents archiving and management
CRC Out-sourcing Service




Experienced team, efficient management processes
MedTrials Solutions has own SITE SOPs and a clinical research coordinator (CRC) management team with extensive clinical trial experience. Our managers and Team Leaders have years of experience in site management, overseas clinical trials and clinical operations at global pharmaceutical companies. We fully understand the demands of sponsors.
All members of our highly qualified CRC team hold a Bachelor’s or equivalent degree and higher qualifications in nursing, medicine, pharmaceutical sciences , Life Scienceor other related medical sciences,. Most team members have worked in hospitals and are familiar with the work processes in the hospital environment,
Under our project management service model, we develop a project-specific plan which includes risk management, people management and communication planning and Quality Cantrol – all overseen by our CRC project manager. This establishes a firm foundation for providing high-quality services to sponsors.
Wide team distribution
Our CRC services are distributed across a number of cities, covering many of the provi cities and regions in Maharashtra India where clinical studies are actively conducted. As business needs develop, our service distribution is rapidly extending. The wide geographic allocation of our CRC services not only greatly improves our CRC team’s work efficiency, but also reduces project operating costs on travel.